
Lithium-ion batteries represent the most widely used energy storage medium for mobile demand, such as smartphones, electric vehicles, and renewable energy. Scientists around the world are actively working on devising new ways to make these batteries last longer. Now, a significant breakthrough might triple the energy density of lithium-ion batteries.
The news was reported by a team of researchers at the University of Maryland (UMD), the U.S. Department of Energy’s (DOE) Brookhaven National Laboratory, and the U.S. Army Research Lab. Writing in a new study published in Nature Communications, the researchers described a new cathode material, an engineered form of iron trifluoride (FeF3).
Unlike most cathodes used in commercial energy storage hardware, iron trifluoride is composed of environmentally-friendly and cheap elements. Both iron and fluoride offer inherently higher capacities than traditional cathode materials.
A battery essentially consists of electrochemical cells connected in series or parallel to provide voltage and capacity. Each cell contains a positive (cathode) and negative (anode) electrode, which divided by an electrolytic solution, simply called an electrolyte, with dissociated salt that allows ion transfer between electrodes.
Typically, graphite anodes used in most lithium-ion batteries have a much larger capacity than cathodes. “Cathode materials are always the bottleneck for further improving the energy density of lithium-ion batteries,” said Xiulin Fan, a scientist at UMD and one of the lead authors of the paper.
The materials normally used in lithium-ion batteries are based on intercalation chemistry,” said Enyuan Hu, a chemist at Brookhaven and one of the lead authors of the paper. “This type of chemical reaction is very efficient; however, it only transfers a single electron, so the cathode capacity is limited. Some compounds like FeF3 are capable of transferring multiple electrons through a more complex reaction mechanism, called a conversion reaction.”
Credit: Brookhaven National Laboratory
The FeF3 cathode has the potential to triple the energy density of lithium-ion batteries, according to the researchers. If this potential is ever fully reached, it could accelerate our transition away from fossil fuels to renewable energy. Imagine driving an electric vehicle with three times the range currently available or a home that can store three times as much energy from its solar panels during the night.
Of course, FeF3 cathodes aren’t exactly new to science. However, historically this type of cathode has been plagued by three major complications: poor energy efficiency (hysteresis), a slow reaction rate, and side reactions that can cause poor cycling life.
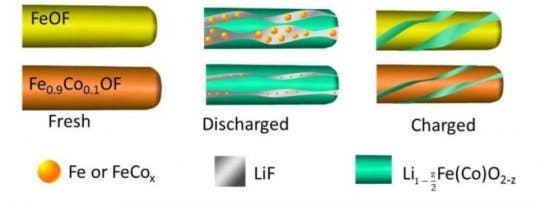
The researchers overcame these challenges by doping the FeF3 nanorods with cobalt and oxygen atoms through a process called chemical substitution. This tweak allowed the researchers to manipulate the reaction pathway of the cathode, making it more “reversible.”
“When lithium ions are inserted into FeF3, the material is converted to iron and lithium fluoride,” said Sooyeon Hwang, a co-author of the paper and a scientist at Brookhaven’s Center for Functional Nanomaterials (CFN). “However, the reaction is not fully reversible. After substituting with cobalt and oxygen, the main framework of the cathode material is better maintained and the reaction becomes more reversible.”
Using a technique called transmission electron microscopy (TEM), researchers at the Center for Functional Nanomaterials (CFN) fired a powerful beam of electrodes on the new cathode nanorods. The imaging experiment, which had a resolution of 0.1 nanometers, revealed the exact size of the nanoparticles that comprise the cathode structure. This step was important, as it showed the researchers that the cathode had a fast reaction speed when changing between different phases of the charge-discharge process.
Unfortunately, TEM can only be used to peer inside a limited area of the sample. So the researchers turned to the National Synchrotron Light Source II (NSLS-II), where they directed the X-ray Powder Diffraction (XPD) beamline through the cathode material. By analyzing how the light scattered, the scientists could “see” additional information about the material’s structure.
Researchers say that their strategy could be applied to other high-energy conversion materials.



