In a most unexpected find, the same University of Manchester team that isolated graphene for the first time in 2003 found that water flattens into square crystals – a never encountered lattice configuration – when squeezed between two layers of graphene. The square ice qualifies as a new crystalline phase of ice, joining 17 others previously discovered. The finding could potentially improve filtration, distillation and desalination processes.
Water, don’t be square
Previously, Andre Geim of the University of Manchester, UK – who shared a Nobel Prize in physics in 2010 for his groundbreaking graphene research – was left scratching his head after he found water vapours could pass through laminated sheets of graphene oxide. This was peculiar since helium couldn’t do this, a molecule that’s a lot smaller than water. To complicate the puzzle, liquid water – which is more tightly bonded than vapor – could also pass through the graphene oxide.
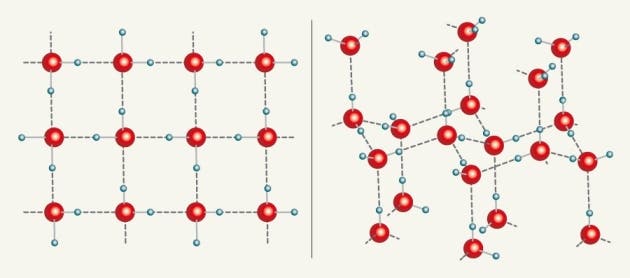
Then, simulations showed that water was forming square ice crystals between the graphene sheets. “But you never trust molecular-dynamics simulations,” says Geim. The team thus proceeded with a simple experiment. They dropped just one milliliter of water on a sheet of graphene (an one atom thick layer carbon arranged in a hexagon lattice), then placed a second one on top. As the water slowly evaporated, it was reduced to an one atom thick layer (just like the graphene!), all arranged in a square lattice at room temperature.
In normal conditions (temperature and pressure), the water molecule has a V shape, with the two hydrogen atoms bonded to the oxygen atom at a 105° angle. Imagine Mickey Mouse, that’s water! In ice form, four bonds are usually arranged in a tetrahedral (pyramid) shape. In the square ice, however, all the atoms line up with a right angle between each oxygen–hydrogen bond.
After several iterations of the experiment, Geim’s team ended up with one, two or three atom thick layers of square ice crystals, all aligned one atop another. Remember, I mentioned the water molecules were squeezed by the graphene. In fact, the pressure exerted by the two layers could be more than 10,000 times that of atmospheric pressure, according to the paper published in Nature. This happens because as the graphene sheets get closer, they distort each others’ electron cloud. The sheets are attracted to one another by a huge intermolecular force known as the van der Waals force, like “having millions of little springs holding them together,” according to Alan Soper, a physicist at the Rutherford Appleton Laboratory in Harwell, UK.
This might not be some queer finding confined to a laboratory setting. Square ice might be encountered in nature where enormous pressure is exerted over tight quarters. It just may be that we haven’t found it yet. On a practical level, the square ice method might improve desalination filters based on graphene.
“Finding out how the water behaves in a capillary is a big part of what we need to do to make a good filter,” says Geim. “This is a very important step.”






