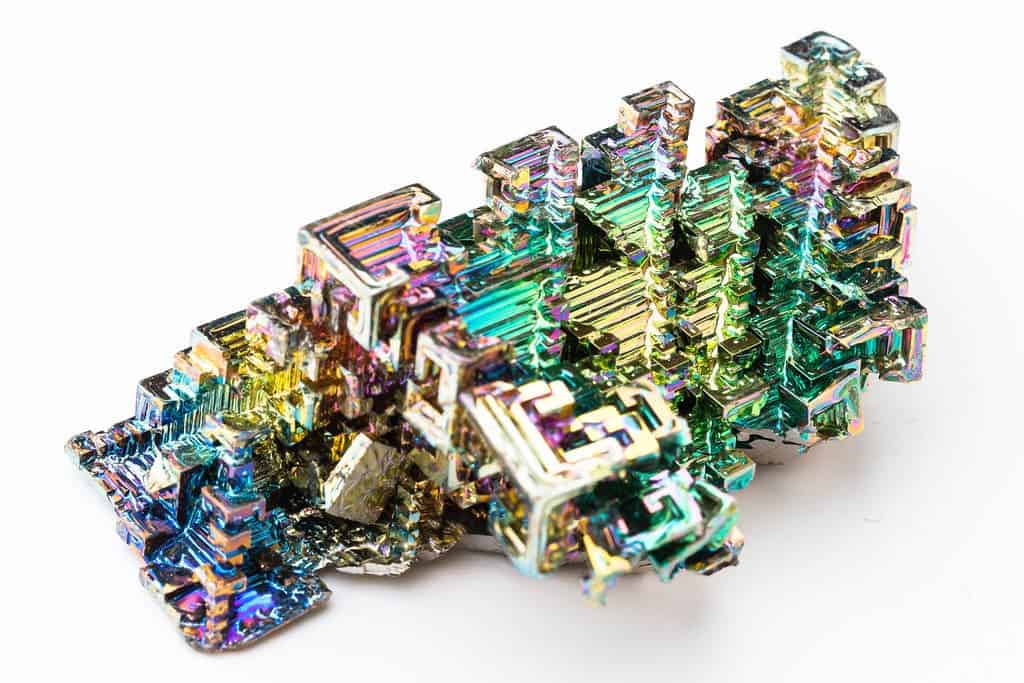
If you happen to see a bismuth crystal, it’s probably shaped unlike anything you’ve ever seen and looks completely unnatural. Draped in iridescent colors, and in seemingly unnatural, geometric shapes, the bismuth crystal is, quite frankly, unlike any other crystal in geology.
But that’s because it isn’t exactly natural.
Bismuth is rarely found in nature in its elemental form. Of growing interest in rock shops, however, are laboratory-grown bismuth crystals. These crystals, while not natural, are nonetheless very interesting to the mineral hobbyist and to others.

What is bismuth
Bismuth is a natural chemical element with the symbol Bi and the atomic number 83. It shows up in the periodic table, where it’s a metal (technically a post-transition metal). It’s got some pretty neat properties, like being one of the most diamagnetic (repelled by magnetic forces) and also one of the least thermally conductive metals known.
Elemental bismuth occurs naturally as a brittle, silvery metal, and in its sulfide or oxide forms (with sulfur or oxygen) it forms important commercial ores. However, it is often covered by a rosy color due to an oxidation layer that forms on its surface. If the bismuth is oxidized even further, it can take on a vividly iridescent appearance (more on that a bit later).
Bismuth was once thought to be the heaviest atom that is not radioactive. However, it was found in 2003 that bismuth actually is radioactive, but it decays at a very, very slow rate. Bismuth’s only isotope decays at such a slow rate that its estimated half-life is a billion times the estimated age of the universe.

Bismuth has been known since ancient times, but in these ancient times, it was often confused with tin and lead due to its resemblance. Even in modern times, it was often mined as a byproduct of the extraction of other metals such as lead or tin, but has received more attention recently because of its properties. The majority of the world’s bismuth (almost 80%) is extracted in China.
But if you’re curious about bismuth crystals and their distinctive shape and color, well, I’ve got some good news and bad news.
The bizarre beauty (and science) of bismuth crystals
The staircase-type crystal is called a hopper crystal (because it resembles a pyramidal hopper container).
The distinctive look of hopper crystals occurs because the edges of the crystals are fully developed, but the interior space is not filled in; it’s as if someone has removed the inside of the crystal. However, the interior just never formed in the first place because the crystal cooled too quickly.
Bismuth isn’t the only mineral that can produce hopper crystals. Several other minerals also produce them, including galena, quartz (called skeletal or fenster crystals), gold, calcite, halite (salt), and water (ice). In fact, salt hopper crystals were described in a scientific paper from as far back as 1912, in salt deposits in Switzerland-Germany. Nevertheless, the mechanism of formation remains imperfectly understood, as a 2018 paper points out.

While hoppering can be natural, this type of crystal is often lab-made. Sorry to burst your bubble, but those pretty bismuth crystals are probably not naturally occurring.
Hoppering can occur when the electrical attraction is higher along the edges of the crystal, which causes the edges to develop faster than the centers. Heating and then cooling the crystal under these conditions can produce very regular hopper crystals.
What makes bismuth extra special among hopper crystals is the oxide layer that forms. This oxide layer interacts differently with different wavelengths of light, creating a dazzling rainbow of colors in addition to the spiral, stair-shaped crystal structure.
But as spectacular as these crystals are, you can probably make your own.
Making bismuth crystals
Right out the gate, here’s a disclaimer: unless you absolutely know what you’re doing, you shouldn’t be cooking bismuth. Seriously.
But if you do know what you’re doing, and you have a piece of bismuth for some reason, you can start with a stainless steel pot or saucepan (the less wide it is, the less bismuth you need to work with).
You can melt bismuth in your pot on the stove, and you should see a hot gray soup-like mass and a thin layer of slag on top. This slag has impurities and has to be removed, either manually (with a fork) or by pouring the bismuth into another metal container (if you want to ruin two pots with this).
Then, make sure to cool the bismuth as slowly as possible — this will make the crystals grow larger. You can leave the metal container on the heat source and gradually reduce and stop it.
The bismuth should start to solidify pretty quickly; you may move it around to prevent it from sticking to the bottom or side of the pan (if it does, you can stick it in the freezer for a moment). You’ll need at least 2 kg (5 pounds) of bismuth to make decently large-ish crystals. Don’t use the metal container for cooking after this!
The crystals won’t be iridescent in the beginning, but it shouldn’t take too long for the crystals to become oxidized. If you’re not happy with the results, you can always reset and start melting the bismuth again.
Bismuth applications (in industry and science)
Bismuth is used in everything from cosmetics and pigments to industrial applications. Bismuth salts are commonly used for gastrointestinal problems — several antacids (such as bismuth subnitrate or bismuth subcarbonate) are used to fight diarrhea, intestinal cramping, constipation, and dyspepsia. Bismuth-based substances are also used to fight some ulcers and gastritis, as well as in surgical packing and pastes used for colostomies. Pepto-Bismol is made of bismuth subsalicylate — a substance with anti-inflammatory, antibacterial, and antacid properties.
Bismuth oxychloride is also used as a pigment for hair sprays, nail polishes, and eye shadows.
In industrial processes, bismuth is often used as a lead replacement. As you may have figured out, bismuth doesn’t have a high toxicity — that’s part of the reason why it’s used as a drug and in cosmetics. But that also makes it a desirable alternative to lead. Bismuth is only slightly lighter than lead, but is far less polluting, which makes it a good replacement. Bismuth managed to find some niche uses in the automotive and aviation industries. Due to its low melting point, it’s also used in things like soldering. All in all, while it’s still nowhere near to other common metals, bismuth has become a mainstay in several areas.
Bismuth is also increasingly used for supercomputers and quantum computers — as it can conduct energy very efficiently, it can keep supercomputers from frying.
In a study, researchers showed that bismuth also has hallmark signatures of materials known as topological insulators, materials that are insulating in their interior but can support the flow of electrons on their surface — a quality that is highly coveted in quantum research.
Bismuth is a pretty special element and makes a pretty special mineral — with a very special crystal. While this crystal is not what makes it so appreciated, it’s definitely a part of what makes bismuth capture the imagination of so many people.






