
Pyrite, commonly known as “Fool’s Gold,” is a fascinating mineral that has captivated humanity for centuries. Its shimmering, metallic luster and pale brass-yellow hue have often led to confusion with gold, hence its well-known nickname. Chemically known as iron sulfide (FeS2), pyrite has some unique properties and widespread occurrence in nature.
Historical Background
Pyrite’s name is derived from the Greek word “πυρίτης λίθος” (pyritēs lithos), meaning ‘stone which strikes fire,’ a nod to its ability to spark when struck against steel. This feature made it valuable in early fire-starting techniques. In ancient Roman times, people used several types of stones that created sparks, with Pliny the Elder’s descriptions suggesting pyrite. By the 16th century, Georgius Agricola, a key figure in the field of early mineralogy, broadened ‘pyrite’ to encompass various sulfide minerals.
The ancient Greeks and Romans used pyrite in jewelry and ornaments. During the Renaissance, pyrite found utility in the nascent field of firearms, particularly in the wheellock mechanism, as it produced the necessary sparks for ignition.
Physical and Chemical Properties of Pyrite
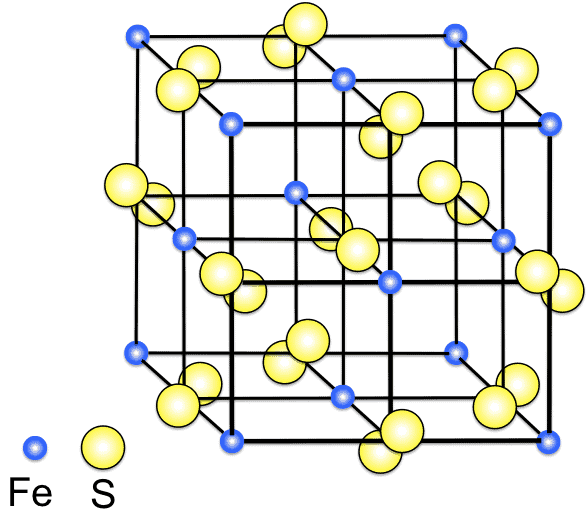
Pyrite typically forms in cubic crystals, often accompanied by striations on the crystal faces. These crystals can also be octahedral and pyritohedral in shape. The specimen’s size can vary greatly, with some examples from notable locations like Huanzala Mine in Peru showcasing impressive dimensions.
Pyrite has a metallic, glistening luster and a pale brass-yellow coloration, which can tarnish to a darker, iridescent tone. Despite its resemblance to gold, Pyrite is distinct in its brittleness and hardness, rating 6 to 6.5 on the Mohs scale of mineral hardness (just a bit softer than quartz, for comparison). This hardness, combined with its uneven fracture and high specific gravity of 4.95-5.10, sets it apart from the malleability of actual gold.
Chemically, Pyrite is an iron sulfide with a simple formula: FeS2. The mineral’s composition leads to some fascinating properties, such as its paramagnetic nature (magnetic susceptibility under the influence of external magnetic fields) and its inability to dissolve in water. Furthermore, Pyrite’s semiconductor properties, with a band gap of 0.95 eV, have opened doors to various scientific and industrial applications.
How pyrite forms
Pyrite commonly forms in igneous, metamorphic, and sedimentary rocks. Its formation is typically linked to hydrothermal processes, where hot, mineral-rich fluids interact with rocks deep within the Earth. In such sulfide-rich environments, sulfur from these fluids combines with iron, giving birth to pyrite. This process is not restricted to high-temperature environments near magma; it can also occur in cooler settings, like within sedimentary deposits.
Adding to this complexity is pyrite’s biogenic formation. In some sedimentary environments, sulfate-reducing bacteria play a crucial role. These bacteria facilitate the reduction of sulfate to sulfide, which then reacts with iron to form pyrite, showcasing a remarkable intersection of biological activity and mineral formation.
Occurrence and distribution
- Quartz Veins and Sedimentary Rock: Pyrite is often found in quartz veins, sedimentary rock, and metamorphic rock. It is particularly common in coal beds and as a replacement mineral in fossils.
- Global Distribution: Significant deposits of Pyrite are found worldwide. Notable locations include the Huanzala Mine in Peru, Navajún in Spain, and large deposits in Rio Tinto, Spain.
- Association with Other Minerals: Pyrite frequently occurs in association with other sulfides or oxides, contributing to diverse mineral assemblages. It’s also found in the sclerites of certain gastropods, showcasing its presence in both terrestrial and marine settings.
Pyrite and gold

Pyrite’s metallic luster and pale brass-yellow hue often lead to its mistaken identity as gold. This superficial resemblance to gold has been both a curse and a boon throughout history, misleading prospectors while also sparking interest in this iron sulfide mineral. But there’s more to it.
It’s not uncommon for pyrite to be found alongside gold, especially in sedimentary deposits. This association has historically been a source of both confusion and intrigue, especially since the mineral has a similar luster to gold. In some cases, pyrite contains small quantities of what is known as ‘invisible gold’ – gold that is incorporated within the pyrite structure itself, adding a layer of mischief to an already confusing mineral. However, the gold content in these cases is typically minuscule.
Despite the visual similarities, pyrite and gold are chemically and physically distinct. Gold is a native element with a chemical symbol of Au and is highly malleable and ductile. In contrast, pyrite is an iron sulfide compound (FeS2) and is notably hard and brittle.
Another key differentiator is the specific gravity. Gold is much denser than pyrite, with a specific gravity of around 19.3, compared to Pyrite’s 4.95–5.10. This means that a piece of gold will feel noticeably heavier than a similarly sized piece of Pyrite.
Modern Uses and Applications for Pyrite
In the industry, pyrite is used in the production of sulfur dioxide, which is crucial in the paper industry and for manufacturing sulfuric acid. This application stems from Pyrite’s thermal decomposition into iron(II) sulfide and elemental sulfur, a process that begins at around 540 °C (1,004 °F).
One of the more recent and exciting applications of Pyrite is in battery technology. It serves as the cathode material in certain types of lithium metal batteries, such as those produced by Energizer. This usage capitalizes on Pyrite’s natural semiconductor properties, which include a band gap of 0.95 eV and its ability to function as an n-type semiconductor.
Pyrite’s potential as a low-cost material for photovoltaic solar panels is actively being explored, with research focusing on synthetic iron sulfide for photovoltaic materials.
In electronics, pyrite has historically been employed in early mineral detectors in radio receivers before the advent of the vacuum tube. Today, it continues to be of interest to crystal radio hobbyists and in certain niche areas of electronics, particularly where its semiconductor properties are advantageous.
Cultural and economic significance of pyrite
In various cultures, pyrite has been regarded as a protective element against evil and negativity. For example, in Thai beliefs, amulets made of pyrite are revered as ‘Khao Tok Phra Ruang’ or ‘Phet na tang,’ considered a sacred item with the power to ward off evil spirits.
Pyrite is valued for its role in the production of sulfuric acid and in the paper industry. China, for instance, is the largest market for imported unroasted iron pyrites, accounting for a substantial percentage of global imports.






