The story of how the moon came to be is likely far more violent than previously thought. Leading astrophysicists believe a massive impact completely vaporized our planet billions of years ago leaving behind a massive cloud of debris that eventually condensed into what we know as Earth and its moon today.
There are many things that suggest the moon was formed from the groins of the Earth following a giant impact with another planetary-sized object. These hints include the moon’s large size relative to that of the Earth and its orbit, the tidal locking (the moon always shows the same face) or the fact minerals from both worlds trap similar isotopes. This is lunar formation theory makes the most sense, but it’s in the fine details that many scientists seem to disagree. The milestone study might put the debate to rest after researchers used a novel analytical technique that’s ten times more accurate than the best previous method to show this ancient impact basically vaporized the planet, the ‘first’ one at least.
The giant impact hypothesis was first laid on the table in the mid-1970s when astrophysicists proposed the moon was formed by a grazing collision between the proto-Earth and a Mars-sized body called Theia. This eventually became the leading hypothesis that explained how our sole natural satellite came to be. But then in 2001, scientists reported that the isotopic compositions of a variety of elements collected from both terrestrial and lunar rocks are nearly identical.
These isotopic findings go against the status quo since numeral simulations for the ‘grazing impact’ hypothesis show 60 to 80 percent of the material that eventually coalesced into the moon came from the impactor rather than Earth. Yet, the chance of two planetary bodies formed in different parts of the solar system having the same isotopic compositions is tiny. This contradiction has left many scratching their heads and back to the drawing board because the probability the impactor happened to have the same isotopic signature as Earth was just too small to leave it like that.
At first, scientists thought more data would help solve this conundrum but as more data pilled up it only confirmed that the Earth’s and moon’s isotopic signatures are not distinguishable.
“These are the most precise measurements we can make, and they’re still identical,” said Washington University in St. Louis geochemist Kun Wang.
“So people decided to change the giant impact hypothesis,” Wang said. “The goal was to find a way to make the Moon mostly from the Earth rather than mostly from the impactor. There are many new models — everyone is trying to come up with one — but two have been very influential.”
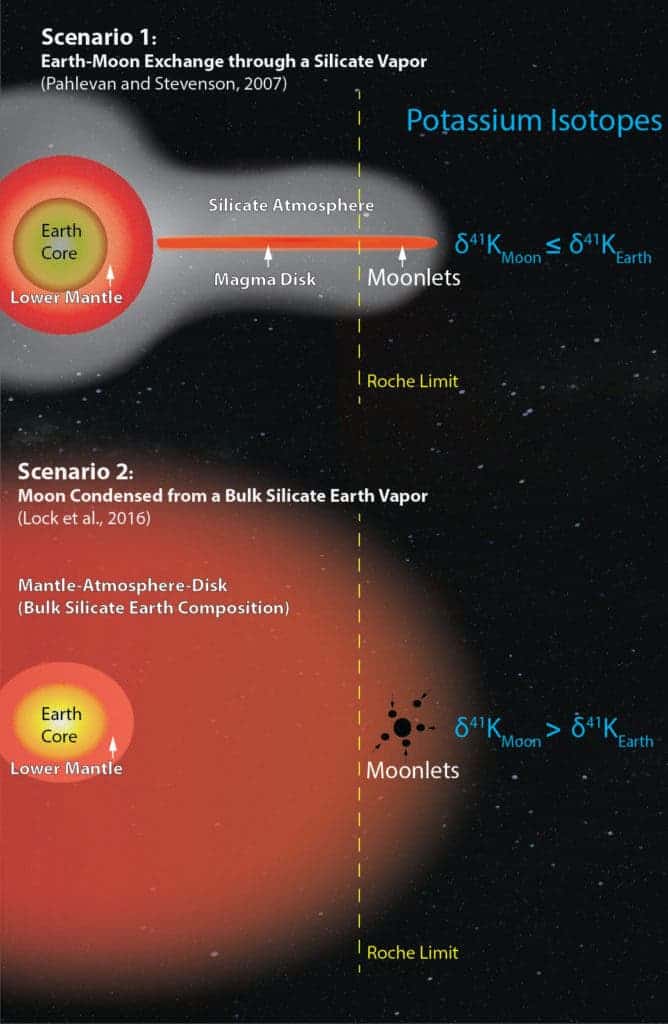
The two new models Wang alludes to are:
- The Silicate Vapor Atmosphere hypothesis. The leading lunar formation theory says the impact melted a part of the Earth and the entire impactor (Theia). Like clay from a potter’s wheel, some of this melted material was flung outward and ‘tada!’ we have the moon. In 2007, a group of astrophysicists spiced things up by adding a silicate vapor atmosphere around the Earth and the residual magma disk that was left of the impactor. The silica vapor provided the necessary exchange medium between the Earth and the material in the disk before the moon got the chance to form.
- The Total Vaporization hypothesis. In 2015, scientists proposed the impact was far more violent. So violent, in fact, that both the proto-Earth and impactor’s mantles vaporized and mixed together to form a dense vapor mantle haze which occupied a space more than 500 times bigger than today’s Earth. As this atmosphere cooled, the Moon condensed from it.
Wang is of the opinion that the silicate vapor atmosphere exchange rate is too slow to explain the same isotopic signatures. Instead, only the thorough mixing of the two bodies explains the identical isotope composition of the Earth and Moon.
Wang and colleagues put the total vaporization event to the test. The team examined seven lunar rock samples collected from different lunar missions and compared their potassium isotope ratios to those of eight terrestrial rocks representative of Earth’s mantle. For this study two of potassium’s three stable isotopes, potassium-41 and potassium-39, since these are abundant enough to measure with precision.
The analysis published in Nature shows lunar rocks were enriched by about 0.4 parts per thousand in the heavier isotope, potassium-41. The only process that would lead to this sort of event, say the researchers, is incomplete condensation of the potassium from the vapor phase during the moon’s formation.
If this process happened in absolute vacuum, the enrichment of heavy potassium isotopes in the lunar samples should be about 100 parts per thousand, which is much higher than what we observe. However, Wang says high pressure can suppress the fractionation of the isotopes. Calculations suggest that the observed enrichment can be obtained if the moon condensed in a pressure of 10 bars or more, roughly 10 times the sea-level atmospheric pressure on Earth today.
The silicate atmosphere model predicts lunar rocks ought to contain less of the heavier potassium isotope than lunar rocks but Wang’s study found the opposite may be true.
Billions of years later, lone potassium isotopes seem to recount tumultuous times packed with cosmic violence. The story of how the moon came to be is far from complete but it does seem to get more interesting.
Was this helpful?



