A research team of scientists from the University of Vienna and the University of Basel have tested the principle of quantum superposition on an unprecedented scale. The team brought hot, complex molecules comprised of approximately 2,000 atoms into quantum superposition and caused them to interfere.
This confirms quantum phenomena can occur on a mass-scale never achieved before, resulting in new constraints being placed on alternative theories to quantum mechanics.
Markus Arndt is a Professor of Quantum Nanophysics at the University of Vienna and led the research team. Their results are published in the journal Nature Physics. He explains the principle of superposition:
“While in classical mechanics you describe bodies with momentum and position, quantum mechanics tells us that matter has to be described by a wave function.
“The amplitude squared of this wave function tells us where to find a particle.”
The principle of superposition emerges from one of the fundamental elements of quantum mechanics — the Schrodinger equation. Drawing an analogy to water waves, this means that these ‘quantum waves’ — or de Broglie Waves named after French Physicist Louis De Broglie — can exhibit constructive and destructive interference effects. The major difference is, whilst the water wave contains a multitude of particles, the de Broglie wave describes a single particle.
Arndt continues the analogy: “Like water waves, the quantum matter-waves can fill large areas of space, this means that a particle has no well-defined position anymore.
“Colloquially we say that the particle can be in two or more places at once. Whilst in free propagation the wave function collects information about places that a classical billiard ball could never know. ”
When a measurement is made the particle can only be measured in one place, a behaviour described as the collapse of the wavefunction. The most famous and familiar demonstration of this effect is the double-slit experiment.
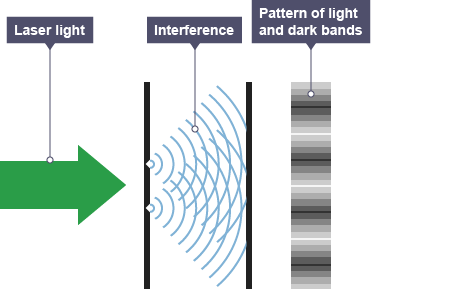
The experiment was initially run with photons — unveiling that they possess both wave-like and particle-like properties [you’ll often see this described as light ‘being both a particle and a wave’ which is incorrect. It’s actually neither].
This particle-wave duality nature was later discovered in matter particles by re-running the experiment with particles of increasing mass — first electrons — right up to carbon-60 molecules, also known as Bucky Balls.
This wave-like aspect of nature is clearly an aspect of quantum mechanics and the very small — but not something we see in the world around us. This leads researchers to an interesting question: where does the boundary between quantum and classical mechanics lie?
Or alternatively, how large does a billiard ball have to be before it can’t be described with the mathematics of waves anymore?
Where is the quantum/classical boundary?
Arndt and the team demonstrated quantum interference with larger objects than ever attempted before. The previous largest molecule used for such experiments weighed in at 10.123 atomic mass units (amu), whilst the molecules that Arndt and his team used were over 2.5 x 10⁴ amu — greater by a magnitude of 100 — the researcher tells me.
One of the largest molecules the team sent through their interferometer — C707H260F908N16S53Zn4 — consists of more than 4.0 x 10⁴ protons, neutrons and electrons. Its de Broglie wavelength is a thousand times smaller than the diameter of a hydrogen atom.
These molecules were specially created for the experiment by Marcel Mayor and his team at the University of Basel. Their technique makes the molecules stable enough to form a beam of molecules in an ultra-high vacuum.
The matter-wave interferometer in Vienna that the team used was specially designed with a two-metre-long baseline, in order to make it adept at highlighting the quantum nature of the particles in question. They also have exciting potential future applications.
“These interferometers that we build for these foundational questions, are exquisite force sensors,” Arndt explains. “Applied to biomolecules or cluster we use them to learn about the internal properties of these particles, even though quantum mechanics forbids us to know where they are.”
The team calls this matter-wave interference assisted metrology and are still the only group in the world working on it, Arndt says.
Living on the edge. Probing quantum physics boundaries
By showing that a superposition can be maintained for a massive particle, Arndt and his team have effectively placed important boundary conditions on a class of models aimed to define that transition from quantum to classical mechanics.
Arndt explains why models that tie mass to the collapse of superposition — legitimate mathematical extensions to Schrodinger’s equation — are appealing:
“Continuous spontaneous localization models need this to explain why small things behave quantum mechanically and big ones don’t.
“ The Schrödinger equation depends on derivatives by space and time. If a particle curves space-time in different places, how can one still have a consistent Schrödinger equation?”
As for where that boundary lies, Arndt believes that the researchers aren’t quite at that limit just yet.
“It’s hard to say. It’s a matter of experimental control above all,” he explains. “There is good reason to believe that if we do it right, we will see quantum effects for quite a while yet.”
The crux of the challenge of finding where quantum effects cease could lie in the discovery of a quantum theory of gravity.
Arndt elaborates: “There is a well-founded suspicion that something may change at high masses because gravity deforms space-time and the particles themselves become a source for that.
“When exactly this may happen, no one can say with certainty. There is no quantum gravity theory yet.”
Original research: https://www.nature.com/articles/s41567-019-0663-9





