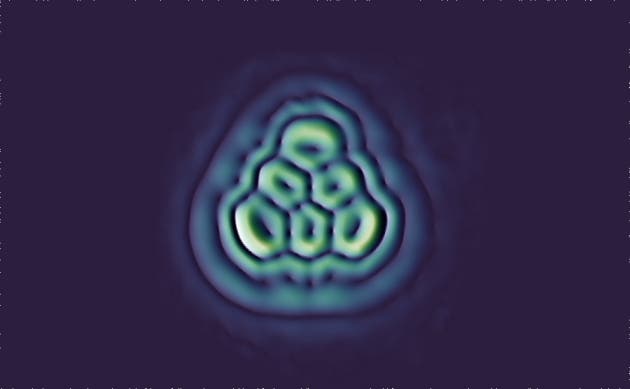
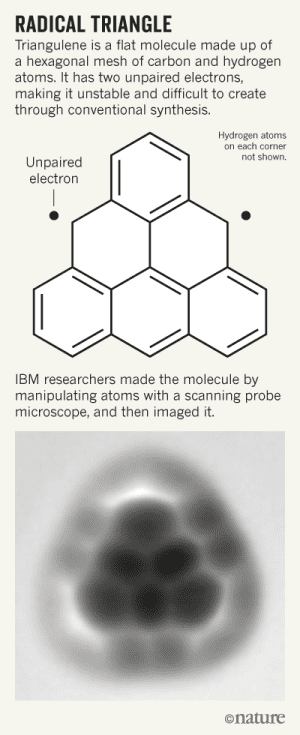
For decades, chemists have been trying to synthesize triangulene — a very unstable molecule that resembles a fragment of graphene. Making the hexagonal mesh of carbon and hydrogen atoms has proven almost impossible to do through conventional means which is why IBM researchers working in Zurich, Switzerland, took a radically different approach. They were able to concoct the elusive molecule by individually placing atoms with a needle-like microscopic tip.
Exotic molecules call for exotic measures
In 1950, Czech chemist Erich Clar claimed that a triangle-shaped hydrocarbon molecule could exist in a configuration of six fused circular benzene molecules. The chemist tried to synthesize triangulene in a solution but failed because it was too reactive. Even though the benzene molecules have an even number of atoms and electrons, two electrons are unable to find a pair because of triangulene’s configuration. It acts like a free radical, basically. “As soon as you synthesize it, it will oxidize,” says Niko Pavliček, a member of the IBM team.
Over the intervening decades, countless other chemists have tried to no avail to synthesize triangulene. There is some progress, as some have been able to synthesize triangulene precursors but not the elusive molecule itself.
Leo Gross and colleagues at IBM labs in Zurich realized there was no point in using conventional synthesis, i.e. reacting chemicals to form larger molecules, so they tried something different. They turned to a scanning probe microscope, an instrument typically used to image molecules by measuring the attractive forces between the tip of the probe and the sample. But the same microscope can be used to direct the course of chemical reactions and synthesize unstable intermediate molecules.
The team started with a precursor called dihydrotriangulene, which lacks the reactive unpaired electrons.The precursor molecules were deposited on a surface which was then probed with the electron microscope.The molecule was then blasted with an electron beam to tear off the extra hydrogen, leaving only the triangulene behind. The same versatile microscope was then used to take this incredible shot, as reported in Nature Nanotechnology.
“Triangulene is the first molecule that we’ve made that chemists have tried hard, and failed, to make already,” said Gross.
It’s thought triangulene’s unique electronic arrangement could make it magnetic and a promising material in quantum computing.
Was this helpful?



