Crystals truly are one of nature’s miracles — not because of some pseudoscientific belief in their ‘healing’ properties, but because of the way the atoms are almost magically aligned into regular structures.
Now, researchers are learning more about how crystals form on different surfaces.

A crystal is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure. Like soldiers standing in formation, the atoms arrange themselves in the most energy-efficient fashion in all three dimensions, sometimes producing stunningly regular and beautiful shapes, which is why crystals are often regarded with fascination and interest.
But crystals are more than just a “pretty face” — they’re very useful in a number of real-life applications.
The process of crystallization (in which the atoms line up in orderly arrays) is the basis of many important materials, including the silicon in microchips and solar cells. But while crystals can form naturally, oftentimes, some man-made materials involve growing crystals on solid surfaces, rather than in a solution. This process is typically very hard to study and there has been surprisingly little research on this topic.
Now, a team of researchers has found a way to reproduce this growth and study it in detail.
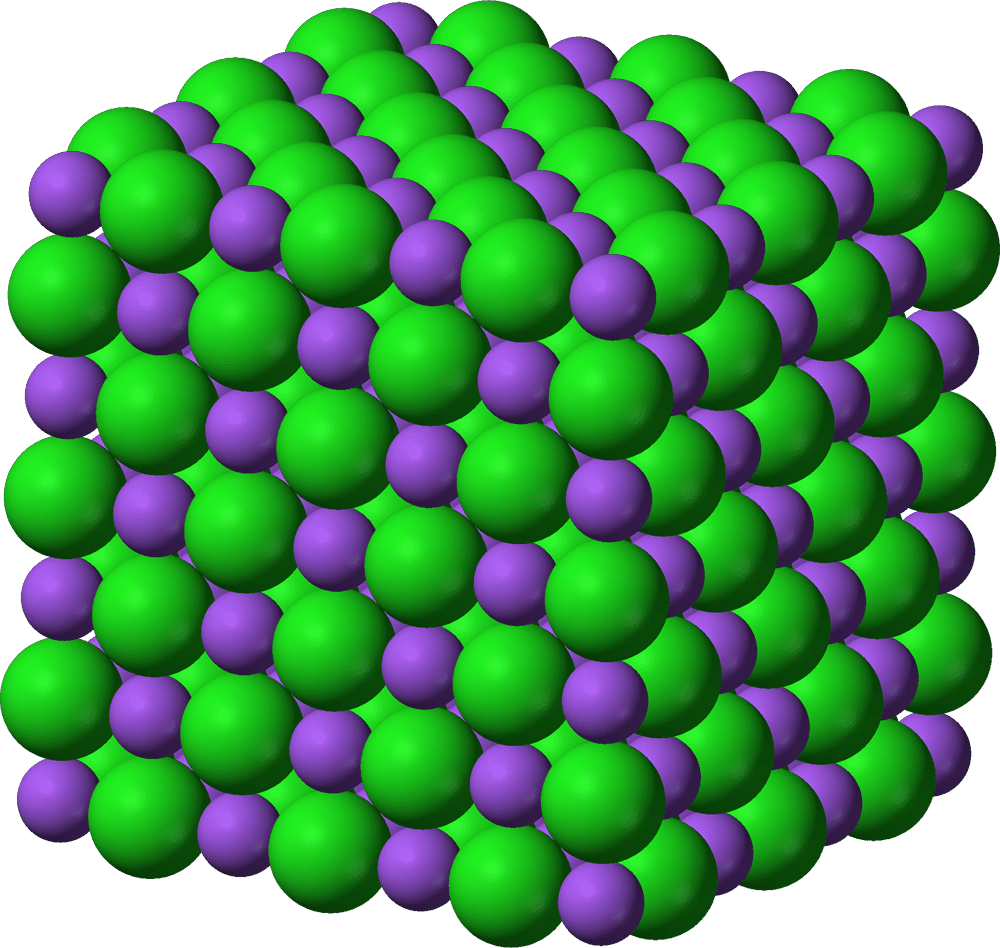
Rather than assembling these crystals from actual atoms, they describe a way to make crystals using “programmable atom equivalents” (or PAEs) — things that behave like atoms in this particular scenario, but are larger than atoms. The key to this process is the fact that the crystal lattice arrangement is based on geometry and doesn’t necessarily rely on any specific chemical or electronic properties of its constituents.
In the study, the team used spherical nanoparticles of gold, coated with strands of DNA. Single DNA strands have the property of attaching themselves to other DNA strands, so it was a surefire way of getting the particles to align themselves in the desired way.
“If I put a very dense brush of DNA on the particle, it’s going to make as many bonds with as many nearest neighbors as it can,” says Robert Macfarlane, one of the study authors. “And if you design everything appropriately and process it correctly, they will form ordered crystal structures.” While that process has been known for some years, this work is the first to apply that principle to study the growth of crystals on surfaces.
The resulting structures are easier to study than conventional crystals because they are approximately 100 times larger. Furthermore, the formation process is also much slower, which makes it easier to analyze in detail.
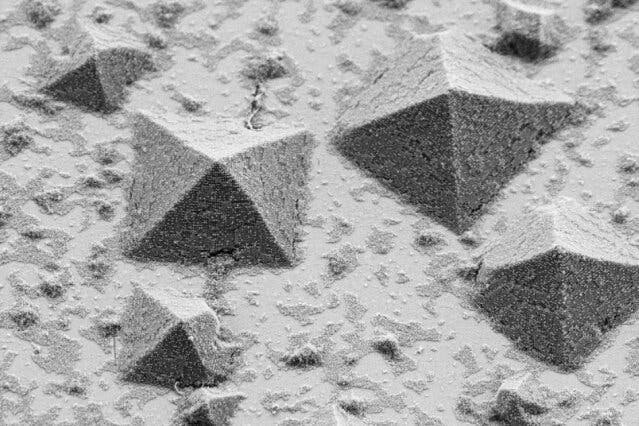
This could enable researchers to better understand and improve crystal-making, tweaking the manufacturing process based on the desired outcome.
“Understanding how crystals grow upward from a surface is incredibly important for a lot of different fields,” he says. The semiconductor industry, for example, is based on the growth of large single-crystal or multi-crystalline materials that must be controlled with great precision, yet the details of the process are difficult to study. That’s why the use of oversized analogs such as the PAEs can be of such benefit.
The only downside to this approach is that creating DNA-coated balls in this way is expensive. As a next step, the Macfarlane lab is looking at ways to reproduce these structures using inexpensive materials.
Journal Reference: Diana J. Lewis et al. Single-crystal Winterbottom constructions of nanoparticle superlattices, Nature Materials (2020). DOI: 10.1038/s41563-020-0643-6









